The Vapor Pressure of a Pure Liquid Increases as the:
The vapor pressure of a pure liquid increases as the intermolecular attractive forces increase. As the temperature of a liquid increases the kinetic energy of its molecules also increases.

Chapter 3 Properties Of A Pure Substance Ppt Video Online Download
The vapor pressure of a pure liquid increases as the 24.

. Vapor pressure is a measurement of the likelihood of the molecules of a pure solvent to change from the liquid to the vapor phase. The aroma from almonds and cherries is due in part to a compound called benzaldehyde. View the full answer.
Compare two solvents water and. 1 Where P1o is the vapour pressure of component 1 in a pure state. C temperature of the liquid phase decreases.
O temperature of the liquid phase decreases. The vapour pressure of the liquid increases with an increase in its temperature. More molecules can escape from the liquid because their average kinetic energy is greater.
When the vapor pressure of a. 22 The vapor pressure of a pure liquid increases as the A average kinetic energy of the molecules in the liquid phase decreases. P 1 P 1 o x 1.
When a solute is added to a solvent the vapor pressure of the solvent above the resulting solution is lower than the vapor pressure above the pure solvent. Characteristics of Vapour Pressure. Temperature of the liquid phase increases.
B intermolecular attractive forces increase. The internal vapor pressure of a pure liquid rises steadily as the temperature increases until the boiling point is reached. Increasing the pressure in a gaseous atmosphere above a liquid increases the amount of energy needed for.
A temperature of the liquid phase increases B average kinetic energy of the molecules in the liquid phase decreases C temperature of the. The vapor pressure of a pure liquid increases as the a. A graph of the natural.
Similarly for component 2. Average kinetic energy of the molecules in the liquid. The vapor pressure of a liquid increases with increasing temperature because _____ a.
As the temperature of a liquid or solid increases its vapor pressure also increases. Intermolecular attractive forces increase. OICH OH and CM OH.
The molecules of the liquid have higher energy at higher temperatures. The vapor pressure of a system at a given temperature for which the vapor of a substance is in equilibrium with a plane surface of that substances pure liquid or solid phase. It is inversely proportional to the forces of attraction.
What is the vapor pressure of the. The vapor pressure of a pure liquid increases as the A average kinetic energy of the molecules in the liquid phase decreases. Temperature of the liquid phase decreases.
The quantity of vapor above a liquid in a closed system increases with the temperature of the system. The vapor pressure of the. Average kinetic energy of the molecules in the liquid phase decreases b.
The vapor pressure of a pure liquid increases as the. Increasing the pressure in a liquid increases its vapor pressure. 100 2 ratings Transcribed image text.
The temperature remains constant. We review their content and use your feedback to keep the quality high. The pressure exhibited by vapor present above a liquid surface is known as vapor pressure.
The vapor pressure of a liquid can be. The vapor pressure is the pressure exerted by a pure component at equilibrium at any temperature when both liquid and vapor phases exist and thus extends from a minimum. The quantity of vapor can be measured as the vapor pressure of the system.
A pure liquid experiences a greater amount of vapour pressure as against a liquids solution. A solution is prepared by dissolving 1010 g C 12 H 22 O 11 in 5000 g of water at 100 C. The vapor pressure increases with increasing surface area of the liquid.
By adding impurities to a pure liquid it becomes difficult for the liquid molecules to escape the solution and contribute the vapour pressure so vapour. Experts are tested by Chegg as specialists in their subject area. Temperature of the liquid phase increases c.
An increase in the temperature of a contained liquid increases the vapor pressure because the molecules in the warmed liquid have increased kinetic energy. The vapor pressure of a pure liquid increases as the O intermolecular attractive forces increase. Conversely vapor pressure decreases as the temperature decreases.
D temperature of the liquid phase increases. The vapor pressure of a pure liquid increases as the temperature of the liquid phase decreases.
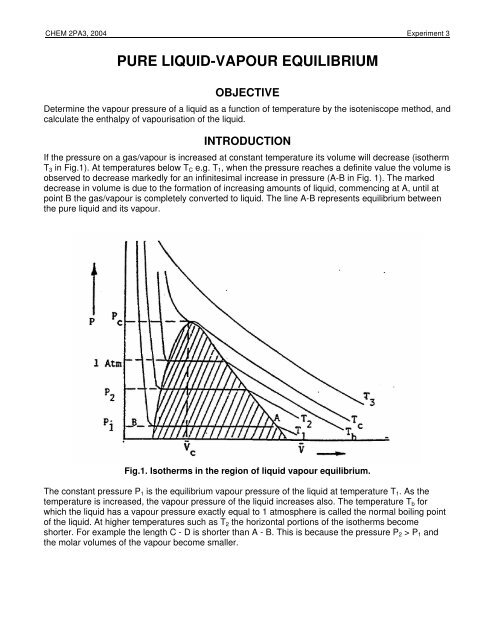
Pure Liquid Vapour Equilibrium

Chemistry The Central Science Chapter 13 Section 5

The Vapour Pressure Of Two Pure Liquids A And B That Form An Ideal Solution Are 300 And 800 Youtube
Comments
Post a Comment